Use of plastic scaffolds seeded with stem cells is no longer strictly stuff of science fiction. The concept has moved from the research lab to human surgeries. Two American companies developed critical technologies for the world’s first and second successful laryngotracheal implants using stem cells taken from the patients’ own bone marrow. The patients’ bodies accepted the transplants without use of immunosuppressive drugs because the patients’ own stem cells were used.
The surgeries took place at Krasnodar Regional Hospital in Krasnodar, Russia. The patients were a 33-year-old mother from St. Petersburg and a 28-year-old man from Rostov-on-Don, who were in auto accidents and suffered from a narrowing of the laryngotracheal junction. Previous surgeries to repair the problem failed. Soon after transplantation, both patients were said to be able to speak and breathe normally.
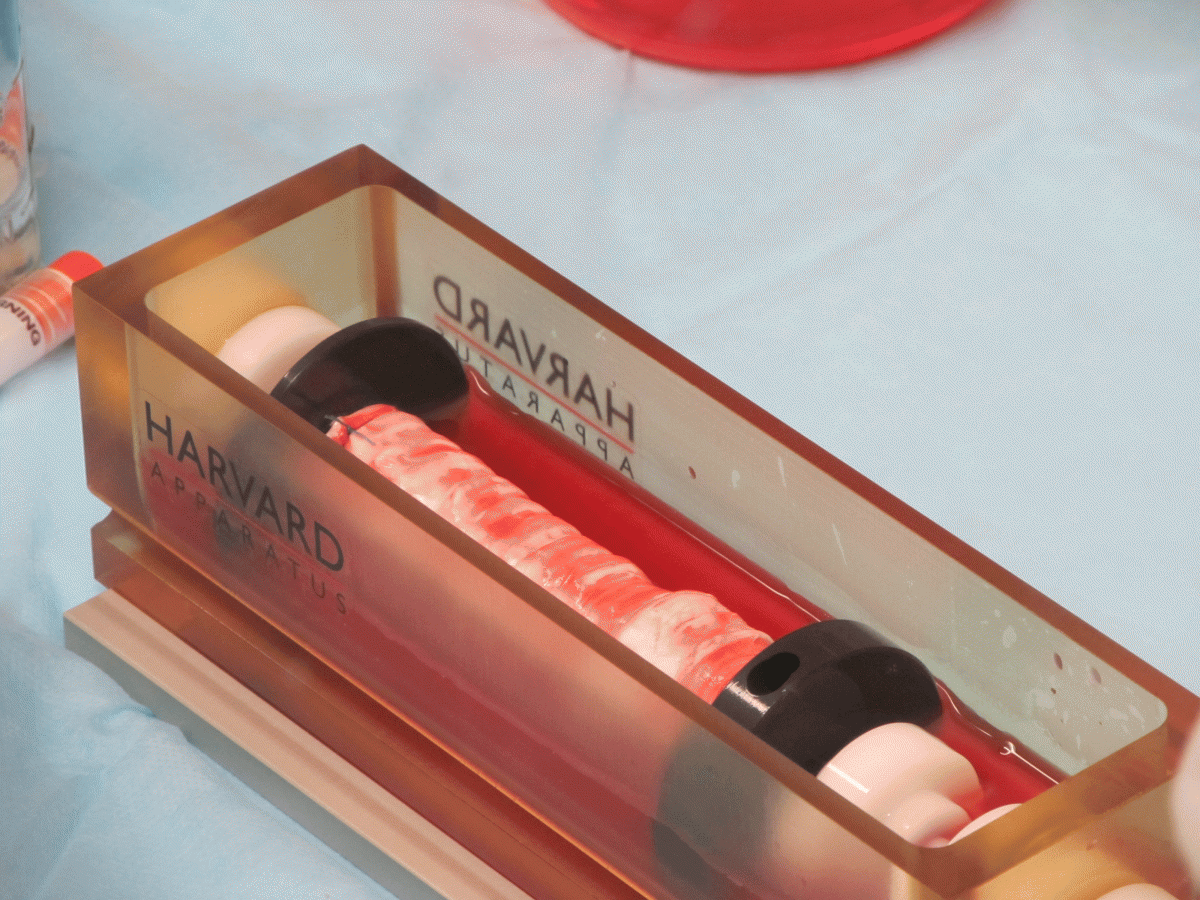
Nanofiber Solutions (Columbus, OH) designed and built the nanofiber laryngotracheal scaffolds to match the dimensions of each patient’s natural larynx and trachea, while Harvard Bioscience (Holliston, MA) provided a bioreactor used to seed the scaffold with stem cells. Each bioreactor was also specifically adapted by Harvard Bioscience to the clinical requirements for each patient and was manufactured in the shape of the patient’s original organ.
“Tissue engineering and regenerative medicine are exciting fields that hold much promise for effective medical solutions,” said Ross Kayuha, CEO of Nanofiber Solutions. “Our nanofiber scaffolds provide an innovative and ideal platform to create an array of new clinical solutions.” The company uses technology licensed through Ohio State University. Its cell culture products use polymer nanofibers to simulate the 3-D structure of human tissue.
Nanofiber Solutions 3D scaffolds are made of polycaprolactone (PCL) and range in thickness from 200-700µm. The nanofibers can be fully degradable or permanent depending on the polymer. Production of scaffolds for other types of hollow organs, such as intestines, blood vessels and kidneys are in the company’s sites. The Russian procedure represents the world’s first and second successful use of synthetic laryngotracheal implants. It is Nanofiber Solution’s second and third successful organ implants using plastic scaffolds within the last year.
The Russian patients were the first entering a clinical trial on regenerative medicine replacement supported by a Megagrant of the Government of the Russian Federation, which is designed to involve leading worldwide scientists with Russian universities. The transplants, which required more than a half-year of preparation, were performed on the first two patients enrolled in an ongoing clinical trial. The Russian Ministry of Health has approved a clinical protocol for an unlimited number of patients in this trial, all of which will involve trachea procedures.
The bioreactors were developed, manufactured and prepared by teams at Hugo Sachs Elektronik, a German subsidiary of Harvard Bioscience and at Harvard Bioscience.
Key participants were Dr. Vladimir Porhanov, head of Oncological and Thoracic Surgery at Kuban State Medical University (Russia); Dr. Jed Johnson, Nanofiber Solution’s Chief Technology Officer who created the synthetic organs; Harvard Bioscience (Boston, USA) who produced the bioreactor; and Dr. Alessandra Bianco at University of Rome, Tor Vergata, who performed mechanical testing during scaffold development.
The principal aim of the $4.8 million, 2.5-year Russian government Megagrant program is to evaluate the molecular mechanisms and underlying pathways of tissue engineering and cell therapy for regenerating airways and lung tissue, and to carry out studies for the prevention and effective treatment of a wide range of diseases.