The University of Warwick and medical equipment company Medherant have jointly developed the world’s first ibuprofen patch delivering the drug directly through skin to exactly where it is needed at a consistent dose rate.
The partners have found a way to incorporate significant amounts of the drug (up to 30% weight) into the polymer matrix that sticks the patch to the patient’s skin with the drug then being delivered at a steady rate over up to 12 hours.
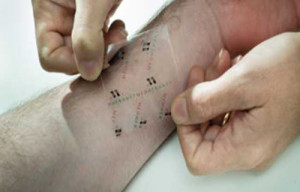
It potentially leads to novel long-acting over-the-counter pain relief products which can be used to treat common painful conditions like chronic back pain, neuralgia and arthritis without the need to take potentially damaging doses of the drug orally.
This novel patch incorporates polymer technology developed by the global adhesive company Bostik and exclusively licensed for transdermal use to Medherant.
According to Medherant, the patch remains highly tacky and thus adheres well to skin even when the drug load reaches levels as high as 30% of the weight/volume of the patch. The drug load made possible by this new technology can be 5-10 times than that found in some currently used medical patches and gels.
The high drug load and consistent drug release profile means the Medherant patches out-perform other patches and gels in their ability to deliver a consistent and significant dose of drug over a prolonged time from a small patch.
“Many commercial patches surprisingly don’t contain any pain relief agents at all, they simply soothe the body by a warming effect. Our technology now means that we can for the first time produce patches that contain effective doses of active ingredients such as ibuprofen for which no patches currently exist. Also, we can improve the drug loading and stickiness of patches containing other active ingredients to improve patient comfort and outcome,” said University of Warwick Research Chemist Professor David Haddleton.
Nigel Davis, CEO of Medherant added, “Our first products will be over-the-counter pain relief patches and through partnering we would expect to have the first of those products on the market in around two years. In addition to our pain relief products, our technology also works with drugs in many other therapeutic areas. We can see considerable opportunities in working with pharmaceutical companies to develop innovative products using our next generation transdermal drug-delivery platform.”
Website: www.adsalecprj.com